Introduction. Chronic myeloid leukemia (CML) is a myeloproliferative disorder with a median age of approximately 60 years. Our study emphasizes the fact that CML can present at younger ages. These patients have significantly different life challenges compared with older patients. The 2020 update of the ELN recommendations mentioned that“ TFR may be the main goal for any patient, irrespective of age, but it is clear that the younger the patient the stronger the case for achieving TFR”. We report here the main characteristics and TFR data in CML young adults.
Methods. The French CML group (FI-LMC) initiated an observational study (CCTIRS no. 14.745) to describe the characteristics of CML in young adult patients in terms of characteristics at diagnosis, treatment choices, tolerance, adherence, therapeutic response, evolution and survival. Patients ranging from 18 to < 30 years with newly diagnosed CML in chronic phase (CP) or accelerated phase (AP) treated with a tyrosine kinase inhibitor (TKI) in first line were eligible for enrolment.
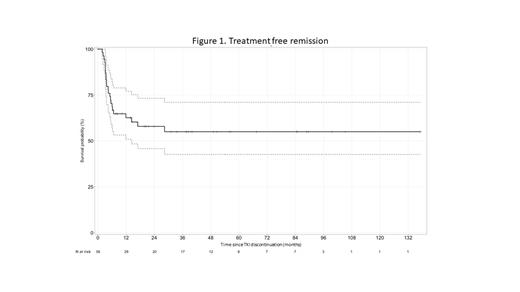
Results. The observatory included 253 unselected young adults from 22 centers with newly diagnosed CML in CP (n=246) or in AP (n=7) among which 51.6% were treated within a first line trial. At the time of CML diagnosis, the median patient age was 24 years (range, 18-29 years); 165 patients were males (63%). Median (range) values were: WBC 154.3 G/L (6.5; 841); platelets 374 G/L (51; 1799), hemoglobin 11.3 g/dL (4.1; 18.3), blast cells 1 % (0; 20), spleen below costal margin 4 cm (0; 30). In CP patients, Sokal score was low in 132 (53.6%) patients, intermediate in 46 (18.7%), high in 37 (15.1%) and unknown in 31 (12.6%) and ELTS score was low in 142 (57.7%) patients, intermediate in 48 (19.5%), high in 22 (9%) and unknown in 34 (13.8%). Initial TKI was imatinib alone (52.4%) or in combination with IFN (2.4%) or Ara-C (0.8%), nilotinib alone (24.6%) or in combination with IFN (4%), dasatinib alone (8.2%) or in combination with IFN (0.4%), bosutinib (1.2%), ponatinib (4.8%) or asciminib (1.2%). Median follow-up is 106.2 months (range: 0.03; 279.2). Ninety-nine (39.3%) patients remained under first-line TKI at latest follow-up and 21 pts (8.3%) underwent bone marrow transplantation (BMT) for resistance or transformation. Median overall survival at 9 years is 96.9% [95% CI: 94.5- 99.4]. Event free survival without death, transformation, or BMT at 9 years is 93.1% [95% CI: 89.7- 96.7]. Six patients died, 1 from sarcoma, 5 from CML including 1 before any therapy (brain hemorrhage). We investigated the TFR strategy. In 169 patients treated for at least 5 years, 56 (33.1%) attempted TKI discontinuation. TKI free survival at 24 months is 57.9% [95% CI: 45.8- 73.2] (Figure 1). Complete analysis comparing characteristics and outcome between the 2 groups of patients (TFR vs no TFR), will be available for ASH presentation.
Conclusion. Despite aggressive features including prominent splenomegaly, high WBC counts and high blast cell percentage, TFR seems achievable in young adults with CML.
Disclosures
Legros:Correspondances en Hématologie: Consultancy, Honoraria, Speakers Bureau; BMS: Honoraria; Incyte Biosciences: Honoraria; NOVARTIS: Honoraria, Other; PFIZER: Honoraria; AMGEN: Honoraria. Huguet:NOVARTIS: Honoraria; INCYTE: Honoraria; PFIZER: Honoraria. Nicolini:INCYTE BIOSCIENCES: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; SUN pharma: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees. Rea:Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; INCYTE BIOSCIENCES: Consultancy, Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Berger:NOVARTIS: Research Funding; PFIZER: Consultancy, Research Funding; NOVARTIS: Consultancy, Research Funding; INCYTE: Research Funding. Roy:Incyte biosciences: Honoraria; Novartis: Honoraria, Research Funding, Speakers Bureau; Pfizer: Honoraria; BMS: Honoraria, Research Funding. Guerci Bresler:PFIZER: Honoraria. Etienne:Pfizer: Honoraria; Incyte biosciences: Honoraria; Novartis: Consultancy, Honoraria, Research Funding; BMS: Honoraria. Jourdan:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Honoraria, Membership on an entity's Board of Directors or advisory committees. Johnson-Ansah:PFIZER: Consultancy; GILEAD: Consultancy; NOVARTIS: Consultancy, Honoraria. Coiteux:Pfizer: Honoraria; Incyte Biosciences: Honoraria, Speakers Bureau; Novartis: Honoraria. Machet:novartis: Honoraria; pfizer: Honoraria.